Chlorine Dioxide Basics...Part 4
An Introduction to the Unique Characteristics of Chlorine Dioxide
Disinfection through Oxidation:
Atoms are composed of matter with electrically charged particles: Protons (+), Electrons (-) and Neutrons (0). The class of biocides referred to as "oxidizers" all work by means of an electron exchange - one atom gains and the other atom loses an electron. All oxidizers may be measured by means of their 'oxidation strength' and their 'oxidation capacity' or 'potential'. The higher the 'strength' the more powerful the attack, the higher the 'capacity' the greater the longevity. It can be compared to two armies - one with a single large cannon (but just a few cannon balls), and another with several small artillery AND an abundance of ammunition.
ClO2 - Compared to Other Oxidizers:
Let's use these two classifications to make a comparison:
Oxidant Strength Capacity
Ozone 2.07 2 e-
Peroxide 1.78 2 e-
Bleach 1.49 2 e-
Bromide 1.33 2 e-
ClO2 0.95 5 e-
What this means is that of the five compounds in the oxidation class, chlorine dioxide has the lowest capacity to do harm to humans and the highest capacity to do harm to microbial metabolism. From this chart you can see that ozone and peroxide can do twice the damage, but with only a third of the capacity to rob electrons and disarm microbes.
To use our artillery analogy, ClO2 may have the smallest gun among the oxidizers, but 250% more ammunition, targeting is easier and damage to environmental surfaces is greatly reduced.
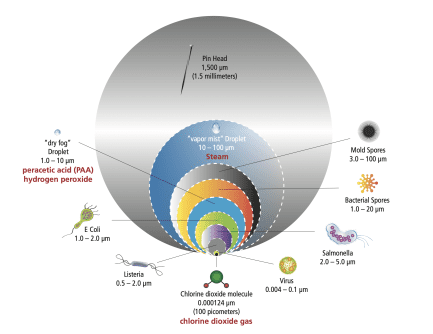
Size Matters:
The very fact that the chlorine dioxide molecule is so small is what makes it so quick and effective. When looking for matter to oxidize, the ClO2 molecule begins with the smallest opponents first - viruses, bacteria (of various sizes), spores, fungi and protozoan. It's strength is exhausted only progressively, and not before it exacts destructive force on the toughest of microbes.
We will cover this in much greater detail with a variety of scholarly reports and laboratory and practical studies.