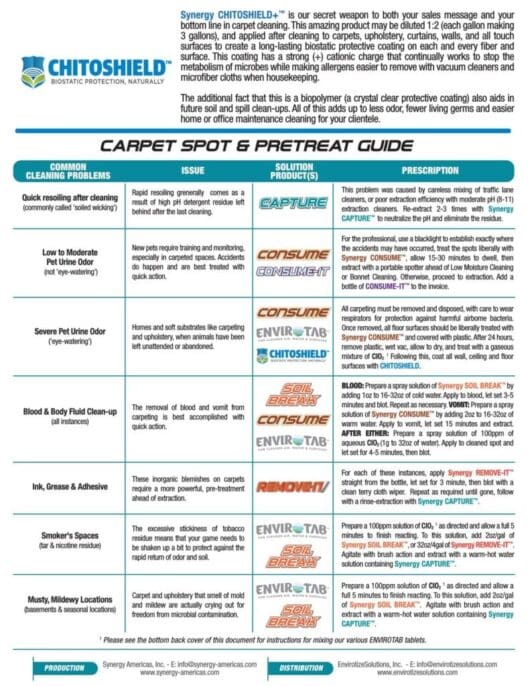
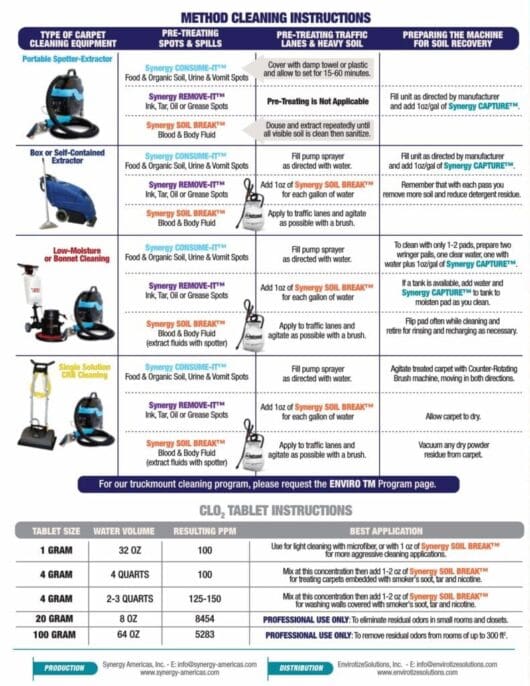
Stay Connected...Drop Us a Line
For best user-experience, hit 'TAB' to move from field to field.
Cleaning Basics...Part 5
What is a Detergent? and
What is a Surfactant?
Listen to the Audio Version:
Soaps versus Detergents:
Soaps, general composed from the fats, oils and lye (or wood ash) have been around for thousands of years. Detergents, made of synthetic ingredients were born out of wartime necessity and have barely pass their centennial birthday.
Synthetic Surfactants:
While simplistically put, detergents have 3 basic components: Surfactants, Solvents & a Vehicle (generally water). The lion's share of the workload is carried on by the synthetic surfactant. These key ingredients have ends that are repellent opposites of one another. One end is hydrophilic (water-loving) and the other is hydrophobic (soil-attracting). This core component both allows the vehicle (water) to spread more evenly, and to convert the water to a soil-carrying vehicle. Solvents (and other lesser ingredients) are placed within these formulas to help to break the bond of the soils from the surfaces.

Other (& More Sustainable) Alternatives:
There are, however, some real downsides to using synthetic surfactants. First, most are petroleum derivatives - not renewably sourced and not readily biodegradable. On the other hand, some surfactants have been derived from plants and hence thoroughly biodegradable. In addition to these alternatives, the employment of microfiber for hard surface cleaning can eliminate the need for any chemical surfactant and the use of chlorine dioxide (for hard surface cleaning) can usually eliminate the need for any other solvent. Beyond detergent residue, biofilm (the scum produced bacteria) is the next main cause for soil build-up on surfaces - and NOTHING breaks through biofilm better than aqueous chlorine dioxide. For more information, see our page, "A Better Way to Clean Hard Surfaces".