Stay Connected...Drop Us a Line
For best user-experience, hit 'TAB' to move from field to field.
Cleaning Basics...Part 9
Using Microfiber to Reduce the Need for Detergents
Listen to the Audio Version:
Detergent Chemistry Review:
Generally speaking, detergents are a synthetic versions of soap. Detergents use an ionic-charged component called "surfactants" (Surface-Active-Agents) to produce a solution that reduces natural water tension, and draws water and other particles together. Sounds pretty good, right?
What is in an ion?
In this case, what ions are doing is carrying and transferring very small magnetic charges from one particle or substance to another, and once bound, they are very difficult to break away. Ionic charges may be innate to a chemical compound, or they may be produced by mechanical action - in this case - friction. Click HERE to read more on ionic surfactants.
How is Microfiber Supreme as a Cleaning Tool?
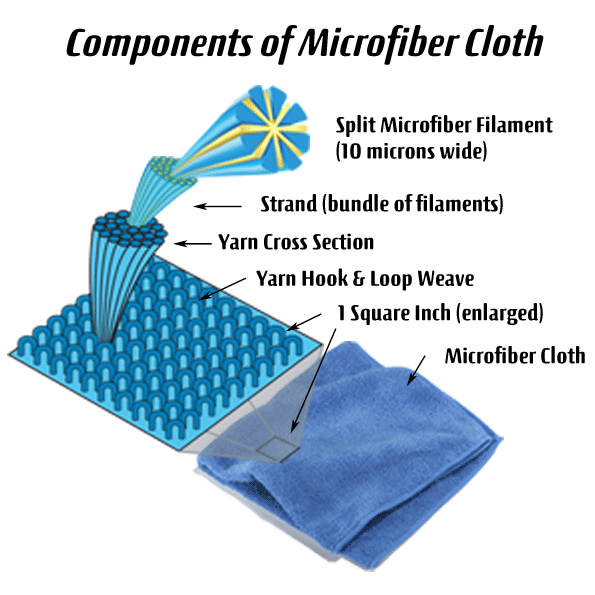
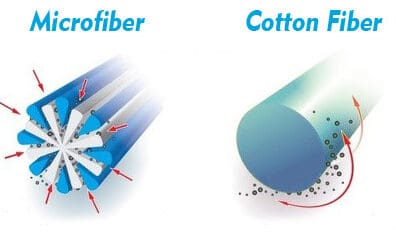
The illustration to the right demonstrates the difference between an extruded microfiber strand and that of a cotton fiber. Unfortunately, the illustration also shows them as being of equivalent size. This is not the case. A strand of microfiber is less than a third the diameter.
Microfiber is also produced of a synthetic material that creates a powerful static-electric charge as it is passed over a surface. So you can certainly visualize that as a microfiber cloth is wiped across a surface, it builds up an attracting charge AND it draws the soil and bioburden into its extruded recesses.
How Can Detergents Ruin Microfiber?
Now please consider the second illustration on the right. Each of these extruded fibers is in a strand, or bundle of filaments, which are then bundled again into a workable yarn containing hundreds of filaments. Each of these are stitched into a tight weave of looped ends that create a tight material for dusting or cleaning surfaces and floors.
If the wrong detergent is used with a microfiber cloth, one that adheres like glue to the extruded surfaces of the yarns, strands and filament fibers, then the material will neither be able to mechanically function to create its charge or to capture its soils.
In short, many general purpose cleaners will repel microfiber from surfaces, so that it cannot scrape soil from the surface, capture and contain it for removal; and ALL detergent-disinfectant (quaternaries) will fill the extruded surfaces with a coating of residue that will fill and kill the cloths ability to do its job.