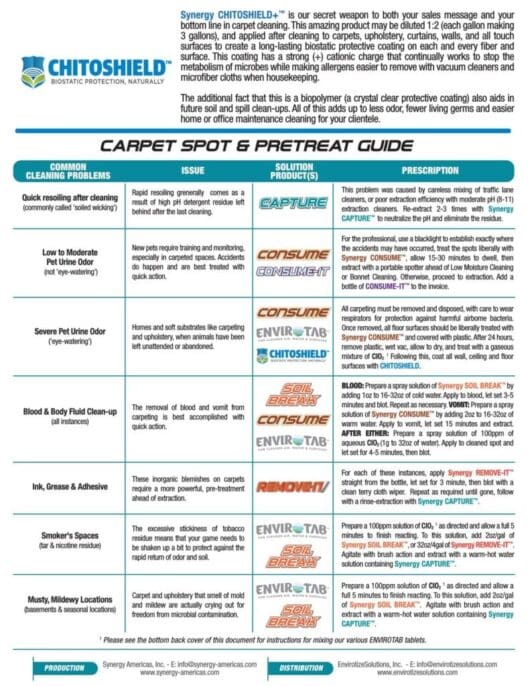
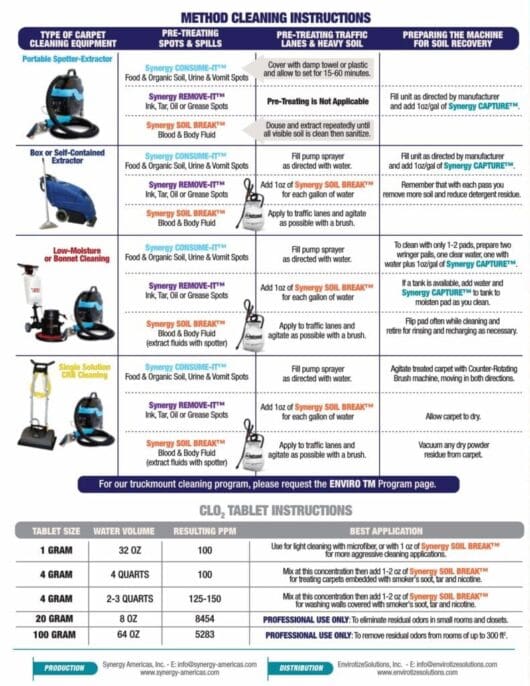
Stay Connected...Drop Us a Line
For best user-experience, hit 'TAB' to move from field to field.
Cleaning Basics...Part 8
A Consideration of HOCl or other Onsite Solutions
Listen to the Audio Version:
The Generation of On-site Generation:
In the past 10 years, there has been a veritable wave of manufacturers offering devices of various sizes that can take plain water and a touch of table salt and generate electrolytic hypochlorous acid for disinfection onsite. Depending on the power of electric consumption, and the percentage of salt added to the water solution, their claims offer some positive and some negative aspects and value. Generally what is happening with this process is the 'splitting' of the water molecule into water plus radical oxygen atom plus radical hydrogen atoms. The hydrogen and the oxygen atoms will then each have strong, but loose ionic charges that attract molecules with opposite charges. Please forgive the raw analogy, but the process can best be illustrated as breaking up a thousand happy homes to create wandering ex-husbands and ex-wives each looking for new mates. The salt added to the water encourages some of these unattached atoms to form a new molecule with a hydrogen atom, an oxygen atom plus an atom of chlorine from the salt. This create Hypochlorous Acid, or HOCl, a weak and unstable aqueous solution.
What is Good about These Devices:
First off, let me state that those units that are most affordable and practical for homes, daycares, schools, clinics and other businesses must be so small that they can only deliver a percentage of active HOCl to create a sanitizer. However, what they are positively providing to the market is a focus upon the "oxidizing" class of disinfectant and moving these same type of facilities away from using off-the-shelf, RTU bottles of liquid poison - quaternaries. This drives down the need for and supply of the 99% water solutions being stocked, stored and shipped as was described in another post.
What is Not-so-Good about These Devices:
As mentioned, these devices electrically convert water and salt into an oxidizing solution. But by necessity, they must also generate another byproduct in the same bottle that robs from the potential of the HOCl, holding it back and continually inviting it to revert to water and salt. In short, the sanitizing power is very short-lived, not easily verifiable, and too often - users are thinking they are sanitizing when in reality they are cleaning with just water.
The Better Solution:
We went another route in our search to eliminate water in shipping, and to create a solution that is an oxidizer which is far more verifiable and stable. The answer is ClO2, and we will explain why in future Cleaning Basics segments.